 Your new post is loading...
 Your new post is loading...
As of today, there are a total of 367 monoclonal antibodies either on the market or in development. 124 are fully human, 146 are humanized, 37 are chimeric, and 56 are murine, or mice antibodies. Of these 367, 38 are FDA approved since the first got through in 1986. 16 of those 38 have been approved in the last 5 years alone, which translates to 42%. We are gaining traction quickly, it seems. Of the 38 approved mabs, 18 are humanized, 14 are fully human, 7 are chimeric, and only one is murine. The difference between the types is this: A chimeric mab is a cross between an animal and a human antibody engineered to react in a human setting. Humanized means that the antibody is mostly converted into a human form but originally came from an animal, usually a mouse. The term "fully human antibody" though is actually a bit misleading because in almost all cases it is not actually taken from a human. It is generally taken from a mechanism called a phage display, where human antibody genes are injected into a phage virus, which then expresses the antibodies on its coat. The best ones are then harvested from the virus. Think of it as microbiological agriculture. Zooming back out to mabs in general, the top 10 bestselling mabs in 2014 account for $68B of the $78B total mab market last year. Number 1 is the fully human adalimumab, or Humira for rheumatoid arthritis, which looks set to eventually overtake Lipitor as the biggest blockbuster drug of all time. Humira was discovered via phage display, along with (nearly) all other fully human mabs on the market or in development.
Via Krishan Maggon
Abstract Enhancing immune responses with immune-modulatory monoclonal antibodies directed to inhibitory immune receptors is a promising modality in cancer therapy. Clinical efficacy has been demonstrated with antibodies blocking inhibitory immune checkpoints such as cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) or PD-1/PD-L1. Treatment with ipilimumab, a fully human CTLA-4–specific mAb, showed durable clinical efficacy in metastatic melanoma; its mechanism of action is, however, only partially understood. This is a study of 29 patients with advanced cutaneous melanoma treated with ipilimumab. We analyzed peripheral blood mononuclear cells (PBMCs) and matched melanoma metastases from 15 patients responding and 14 not responding to ipilimumab by multicolor flow cytometry, antibody-dependent cell-mediated cytotoxicity (ADCC) assay, and immunohistochemistry. PBMCs and matched tumor biopsies were collected 24 h before (i.e., baseline) and up to 4 wk after ipilimumab. Our findings show, to our knowledge for the first time, that ipilimumab can engage ex vivo FcγRIIIA (CD16)-expressing, nonclassical monocytes resulting in ADCC-mediated lysis of regulatory T cells (Tregs). In contrast, classical CD14++CD16−monocytes are unable to do so. Moreover, we show that patients responding to ipilimumab display significantly higher baseline peripheral frequencies of nonclassical monocytes compared with nonresponder patients. In the tumor microenvironment, responders have higher CD68+/CD163+ macrophage ratios at baseline and show decreased Treg infiltration after treatment. Together, our results suggest that anti–CTLA-4 therapy may target Tregs in vivo. Larger translational studies are, however, warranted to substantiate this mechanism of action of ipilimumab in patients.
Via Krishan Maggon
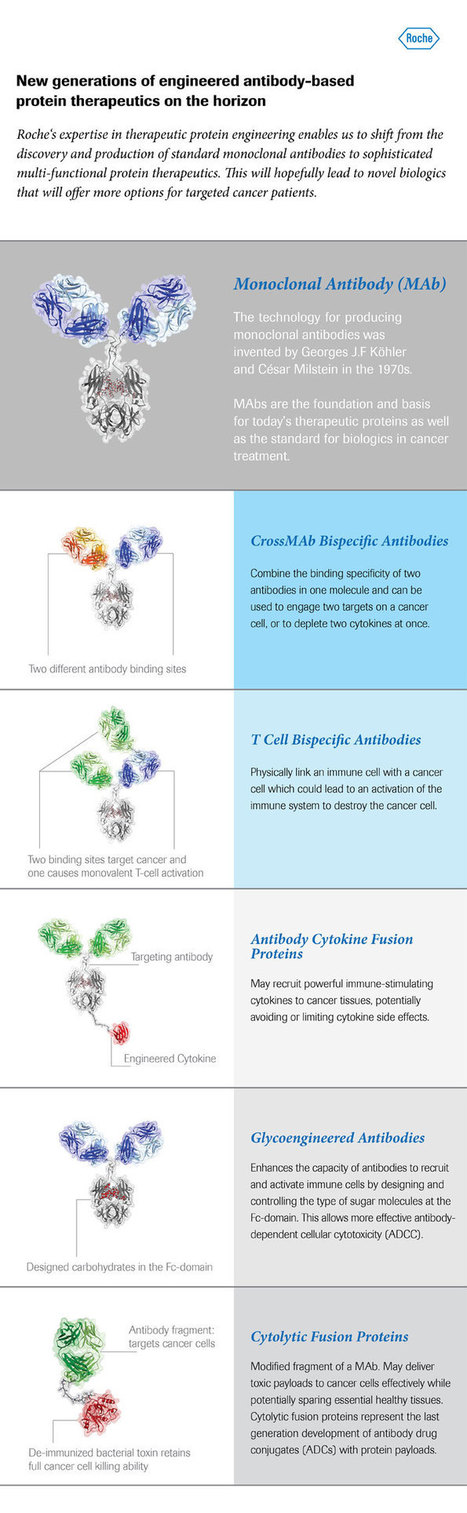
Roche currently has several different therapeutic antibody technologies in early clinical development which in the future could hopefully be a strong pillar in addressing the complexity of targeting more than 250 cancer diseases.
Via Krishan Maggon
(2015). Macrophages are critical effectors of antibody therapies for cancer. mAbs: Vol. 7, No. 2, pp. 303-310.
Via Krishan Maggon
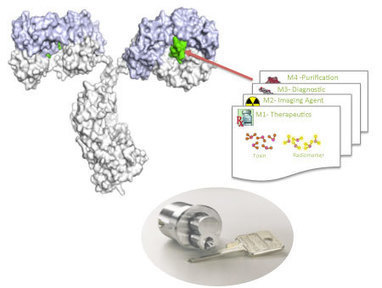
Engineered antibodies are key players in therapy, diagnostics and research.
Via Krishan Maggon
Abstract Antibody-based therapy of various human malignancies has shown efficacy in the past 30 years and is now one of the most successful and leading strategies for targeted treatment of patients harboring hematological malignancies and solid tumors. Antibody–drug conjugates (ADCs) aim to take advantage of the affinity and specificity of monoclonal antibodies (mAbs) to selectively deliver potent cytotoxic drugs to antigen-expressing tumor cells. Key parameters for ADC include choosing the optimal components of the ADC (the antibody, the linker and the cytotoxic drug) and selecting the suitable cell-surface target antigen. Building on the success of recent FDA approval of brentuximab vedotin (Adcetris®) and ado-trastuzumab emtansine (Kadcyla®), ADCs are currently a class of drugs with a robust pipeline with clinical applications that are rapidly expanding. The more ADCs are being evaluated in preclinical models and clinical trials, the clearer are becoming the parameters and the challenges required for their therapeutic success. This rapidly growing knowledge and clinical experience are revealing novel modalities and mechanisms of resistance to ADCs, hence offering plausible solutions to such challenges. Here, we review the key parameters for designing a powerful ADC, focusing on how ADCs are addressing the challenge of multiple drug resistance (MDR) and its rational overcoming.
Via Krishan Maggon
|
Abstract The convergence of advanced understanding of biology with chemistry has led to a resurgence in the development of antibody-drug conjugates (ADCs), especially with two recent product approvals. Design and development of ADCs requires the synergistic combination of the monoclonal antibody, the linker and the payload. Advances in antibody science has enabled identification and generation of high affinity, highly selective, humanized or human antibodies for a given target. Novel linker technologies have been synthesized and highly potent cytotoxic drug payloads have been created. As the first generation of ADCs utilizing lysine and cysteine chemistries moves through the clinic and into commercialization, second generation ADCs involving site specific conjugation technologies are being evaluated and tested. The latter aim to be better characterized and controlled, with wider therapeutic indices as well as improved pharmacokinetic-pharmacodynamic (PK-PD) profiles. ADCs offer some interesting physicochemical properties, due to conjugation itself, and to the (often) hydrophobic payloads that must be considered during their CMC development. New analytical methodologies are required for the ADCs, supplementing those used for the antibody itself. Regulatory filings will be a combination of small molecule and biologics. The regulators have put forth some broad principles but this landscape is still evolving.
Via Krishan Maggon
PASADENA, Calif., April 20, 2015 /PRNewswire/ -- Meditope Biosciences, Inc., a biotechnology company developing novel antibody-based products using its proprietary technology, today announced presentation of data demonstrating the use of its SnAP technology platform for the development of antibody-drug conjugates (ADCs). The data were presented in an abstract at the AACR Annual Meeting 2015, taking place April 18-22 in Philadelphia. Scientists at the company demonstrated the successful transplantation of the meditope binding site to several antibodies. Meditope-peptides of different affinities were then selected by rational design and fine-tuned according to the requirements of specific application. For ADC development, data show that the meditope-peptides are conjugated with cytotoxic payloads and the higher the affinity of the meditope-peptide-drug conjugate for the antibody, the higher the complex's cytotoxic potential against antigen-positive tumor cells.
Via Krishan Maggon
Abstract Antibody-drug conjugates (ADCs) are complex molecules composed of monoclonal antibodies conjugated to potent cytotoxic agents through chemical linkers. Because of this complexity, sponsors have used different approaches for the design of nonclinical studies to support the safety evaluation of ADCs and first-in-human (FIH) dose selection. We analyzed this data with the goal of describing the relationship between nonclinical study results and Phase 1 study outcomes. We summarized the following data from investigational new drug applications (INDs) for ADCs: plasma stability, animal study designs and toxicities, and algorithms used for FIH dose selection. Our review found that selecting a FIH dose that is 1/6th the highest non-severely toxic dose (HNSTD) in cynomolgus monkeys or 1/10th the STD10 in rodents scaled according to body surface area (BSA) generally resulted in the acceptable balance of safety and efficient dose-escalation in a Phase 1 trial. Other approaches may also be acceptable, e.g. 1/10th the HNSTD in monkeys using BSA or 1/10th the NOAEL in monkeys or rodents using body weight for scaling. While the animal data for the vc-MMAE platform yielded variable range of HNSTDs in cynomolgus monkeys, MTDs were in a narrow range in patients, suggesting that for ADCs sharing the same small molecule drug, linker and drug:antibody ratio, prior clinical data can inform the design of a Phase 1 clinical trial.
Via Krishan Maggon
Philochem owns five antibody libraries (ETH2-GOLD, PHILO-1, PHILO-2, PHILO-DIAMOND and PHILOtop) that contain over 30 billion different binding specificities. Philochem’s human antibody libraries (ETH2-GOLD, PHILO-1, PHILO-2 and PHILO-DIAMOND) have been used to generate high-quality binding specificities against >50 different antigens, including human monoclonal antibodies which are currently being developed in clinical trials by Philogen. Philochem’s antibody libraries contain monoclonal antibodies in scFv format. Once monoclonal antibodies which are specific to the target antigen of choice are isolated, they can be easily reformatted into other recombinant antibody formats (e.g., IgG, Fab, mini-antibody, Fc-fusion) using general vectors and well-established procedures which are available at Philochem.
Via Krishan Maggon
Antibody–drug conjugates: targeted weapons against cancer Luisa Iamele, Luca Vecchia, Claudia ScottiDepartment of Molecular Medicine, Unit of Immunology and General Pathology, University of Pavia, Pavia, PV, Italy All authors contributed equally...
Via Krishan Maggon
(2015). The therapeutic monoclonal antibody market. mAbs: Vol. 7, No. 1, pp. 9-14. doi: 10.4161/19420862.2015.989042
Via Krishan Maggon
|



 Your new post is loading...
Your new post is loading...











Humira was the best selling human medicinal brand in 2014 but will be overtaken by Gilead Hepatitis C antiviral sofosbuvir and Harvoni.