Abstract
Objectives The aim of this 12-week Phase IIb study was to assess the efficacy and safety of olokizumab (OKZ), a humanised anti-IL6 monoclonal antibody, in patients with rheumatoid arthritis (RA) with moderate-to-severe disease activity who had previously failed tumour necrosis factor (TNF) inhibitor therapy. The dose-exposure-response relationship for OKZ was also investigated.
Methods Patients were randomised to one of nine treatment arms receiving placebo (PBO) or OKZ (60, 120 or 240 mg) every 4 weeks (Q4W) or every 2 weeks (Q2W), or 8 mg/kg tocilizumab (TCZ) Q4W. The primary endpoint was change from baseline in DAS28(C-reactive protein, CRP) at Week 12. Secondary efficacy endpoints were American College of Rheumatology 20 (ACR20), ACR50 and ACR70 response rates at Week 12. Exploratory analyses included comparisons of OKZ efficacy with TCZ.
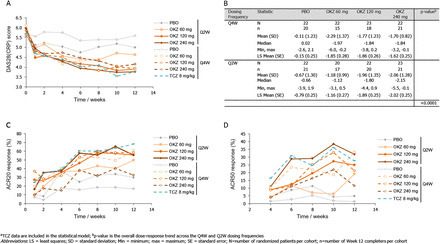
Results Across 221 randomised patients, OKZ treatment produced significantly greater reductions in DAS28(CRP) from baseline levels at Week 12, compared to PBO (p<0.001), at all the OKZ doses tested (60 mg OKZ p=0.0001, 120 and 240 mg OKZ p<0.0001). Additionally, ACR20 and ACR50 responses were numerically higher for OKZ than PBO (ACR20: PBO=17.1–29.9%, OKZ=32.5–60.7%; ACR50: PBO=1.3–4.9%, OKZ=11.5–33.2%). OKZ treatment, at several doses, demonstrated similar efficacy to TCZ across multiple endpoints. Most adverse events were mild or moderate and comparable between OKZ and TCZ treatment groups. Pharmacokinetic/pharmacodynamic modelling demonstrated a shallow dose/exposure response relationship in terms of percentage of patients with DAS28(CRP) <2.6.
Conclusions OKZ produced significantly greater reductions in DAS28(CRP) from baseline at Week 12 compared with PBO. Reported AEs were consistent with the safety profile expected of this class of drug, with no new safety signals identified.
Via Krishan Maggon



 Your new post is loading...
Your new post is loading...




open access full text
Ann Rheum Dis 2014;73:1607-1615 doi:10.1136/annrheumdis-2013-204760
Clinical and epidemiological researchExtended reportEfficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb studyMark C Genovese1, Roy Fleischmann2, Daniel Furst3, Namieta Janssen4, John Carter5,Bhaskar Dasgupta6, Judy Bryson7, Benjamin Duncan7, Wei Zhu7, Costantino Pitzalis8,Patrick Durez9, Kosmas Kretsos10+Author Affiliations
1Division of Immunology and Rheumatology, Stanford University Medical Center, Stanford, USA2Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, USA3Department of Medicine, UCLA, Los Angeles, California, USA4Houston Institute for Clinical Research, Houston, USA5Division of Rheumatology, University of South Florida Health, Tampa, Florida, USA6Department of Rheumatology, Southend University Hospital, Westcliff-on-Sea, UK7UCB Pharma, Raleigh, USA8William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK9Service et Pôle de Rhumatologie, Cliniques Universitaires Saint-Luc, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, Belgium10UCB Pharma, Slough, UKCorrespondence toDr Mark C Genovese, Division of Immunology and Rheumatology, 1000 Welch Road, Palo Alto, CA 94304, USA; genovese@stanford.edu